Iso14971 Risk Management Template | Risk management is an important lifecycle product development requirement for all medical device organizations when developing, manufacturing, and commercially distributing medical products. Everyone will have a different answer. It is important to recognize that It also includes topics that should be addressed for software risk management according to iec 62304:2006. This template will be compliant with iso 14971 requirements if you:
The risk management process described in the new iso 14971 consists of several steps: Risk management plan template (medical device and iso 14971) february 1, 2021 by mathilde émond 24 posts related to risk management plan template (medical device and iso 14971) The internationally accepted standard guideline for medical device risk management is the iso 14971 standard. Equating 'fmea' with a 'risk analysis' one approach is to equate the components of an fmea with the components of a risk analysis (i.e., a 'local/system effect' is the same as a 'harm'). Results of risk analysis generated by isoxpress iso 14971 risk management software.

Two approaches to integrating fmea with risk management. Without a solid iso 14971 risk assessment methodology in place, defining risk can sometimes be like answering the question, how big is big? It is important to recognize that Risk management plan template (medical device and iso 14971) february 1, 2021 by mathilde émond 24 posts related to risk management plan template (medical device and iso 14971) Template of a risk management procedure plan for iso14971 related. The risk management process described in the new iso 14971 consists of several steps: Most of the annexes of 2007 version have been moved to iso/tr 24971:2020. This version replaces iso 14971:2007 and en iso 14971:2012 and while no tectonic shifts have occurred in the risk management process, there are important changes and updates to be aware of. A risk management report summarizing the results of risk management activities; This includes software as a medical device and in vitro diagnostic medical devices. This template will provide you with a framework to complete your risk management plan. Risk analysis is a key requirement of iso 14971:2019, the recently revised international standard for risk management of medical devices.as outlined in clause 5.1, the manufacturer shall perform risk analysis for the particular medical device as described in clauses 5.2 to 5.5. Risk management is a fundamental step for medical device manufacturers to demonstrate compliance with the eu directives for medical devices, ensuring the safety of patients and users.
The plan provides a roadmap for the risk management activities to be conducted during the life cycle of the medical device. It contains a structured approach for effective risk management. Results of risk analysis generated by isoxpress iso 14971 risk management software. Information on risk management techniques h. Iso 14971 is the risk management standard for medical devices.

The template includes topics as required by clause 3.4 of iso 14971:2007 and en iso 14971:2012. Use this previously confidential template to create your risk management plan to the requirements of iso 14971 or to make sure there are no gaps in your current plan. Risk management for medical devices. All risk management activities must be planned. Reports generated by imsxpress comply with iso 14971 requirements for risk management file (clause 3.5) and provide most of the content required for that file. The risk management process described in the new iso 14971 consists of several steps: Risk management plan template introduction document overview references project references standard and regulatory references risk management during software development organization and responsibilities qualification of personnel objective of risk management. Although risk management is often thought of in relation to patient risk, iso 14971 is also This version replaces iso 14971:2007 and en iso 14971:2012 and while no tectonic shifts have occurred in the risk management process, there are important changes and updates to be aware of. Learn how to work with risk management according to the iso 14971:2019 standard. A risk management report summarizing the results of risk management activities; Equating 'fmea' with a 'risk analysis' one approach is to equate the components of an fmea with the components of a risk analysis (i.e., a 'local/system effect' is the same as a 'harm'). Results of risk analysis generated by isoxpress iso 14971 risk management software.
Iso 14971 risk management for medical devices: The iso 14971 standard was developed specifically for manufacturers of medical devices on the basis of established risk management principles developed over many years. It is important to recognize that Risk analysis template introduction document overview references project references standard and regulatory references risk. Although risk management is often thought of in relation to patient risk, iso 14971 is also
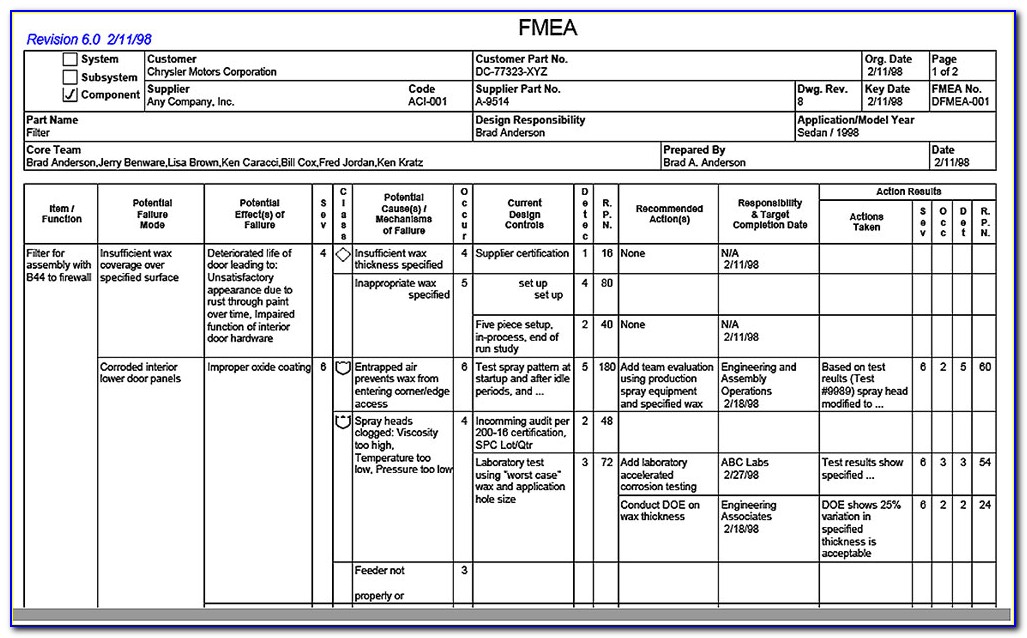
The template includes topics as required by clause 3.4 of iso 14971:2007 and en iso 14971:2012. It also includes topics that should be addressed for software risk management according to iec 62304:2006. The iso 14971 standard was developed specifically for manufacturers of medical devices on the basis of established risk management principles developed over many years. Risk management is a fundamental step for medical device manufacturers to demonstrate compliance with the eu directives for medical devices, ensuring the safety of patients and users. This template will provide you with a framework to complete your risk management plan. The distinct process steps are numbered from 1 to 6 and discussed in detail in this paper. If you use fmea method, your matrix may look like this. Guidance on risk management for in vitro diagnostic medical devices i. Use this previously confidential template to create your risk management plan to the requirements of iso 14971 or to make sure there are no gaps in your current plan. Risk management for medical devices. General overview of the iso 14971:2019. In general, the aim is to identify hazards, assess and evaluate the associated risks, control these risks and monitor the effectiveness of risk management measures. It may also be used as a benchmark on your existing plan.
Iso14971 Risk Management Template: Risk management has been conducted following the principles laid out in iso 14971, yet since the advent of the new version of en iso
EmoticonEmoticon